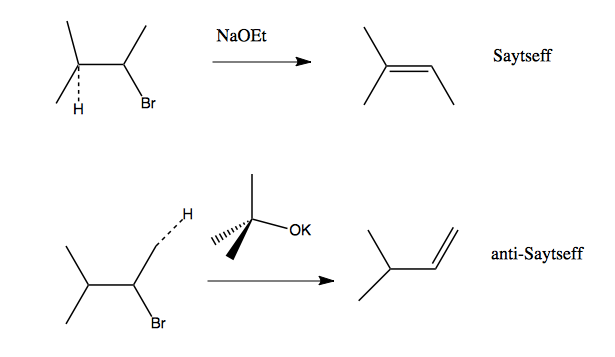
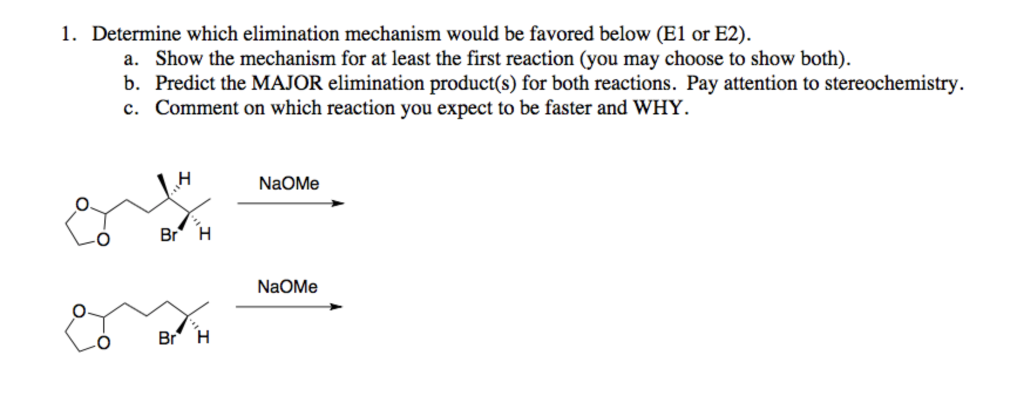
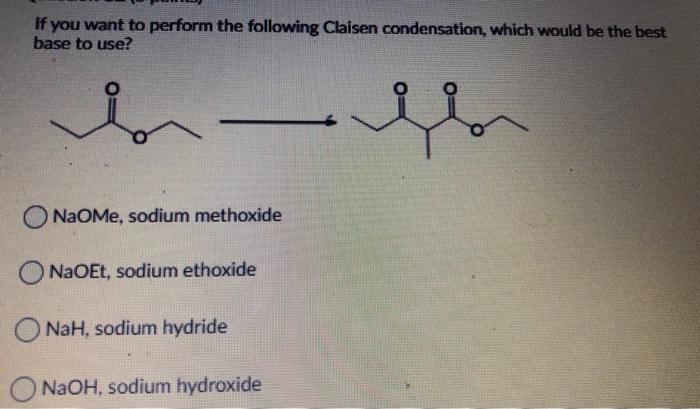
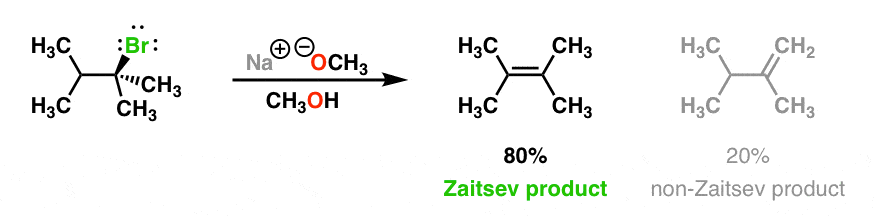
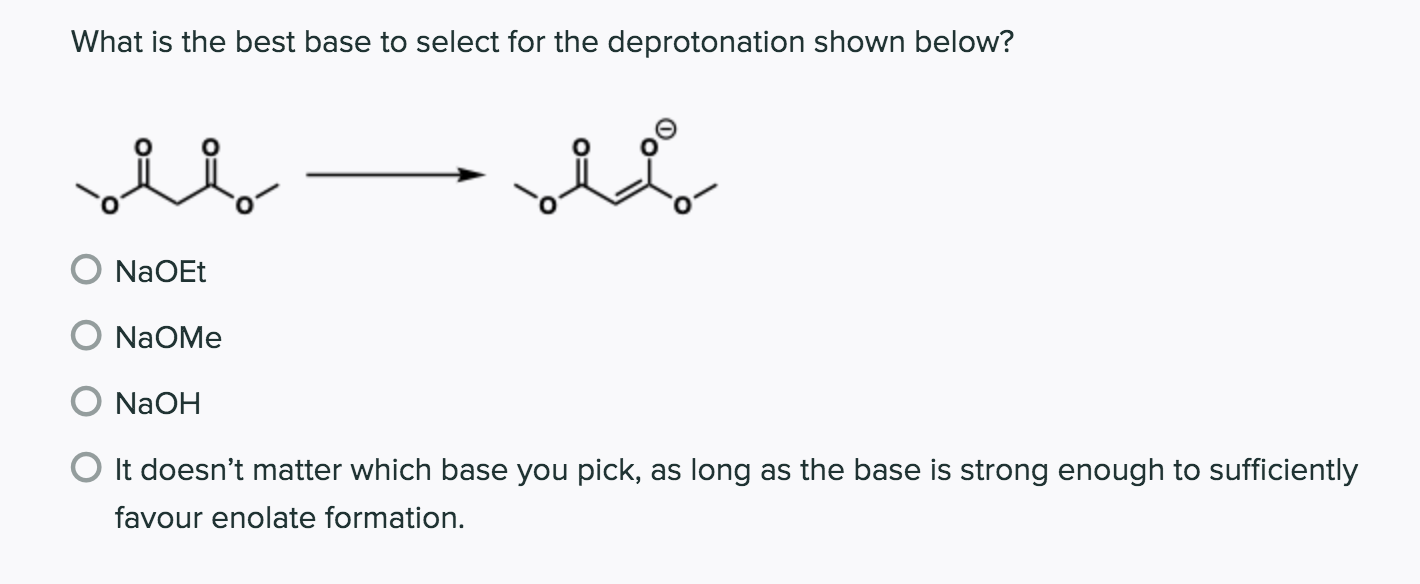
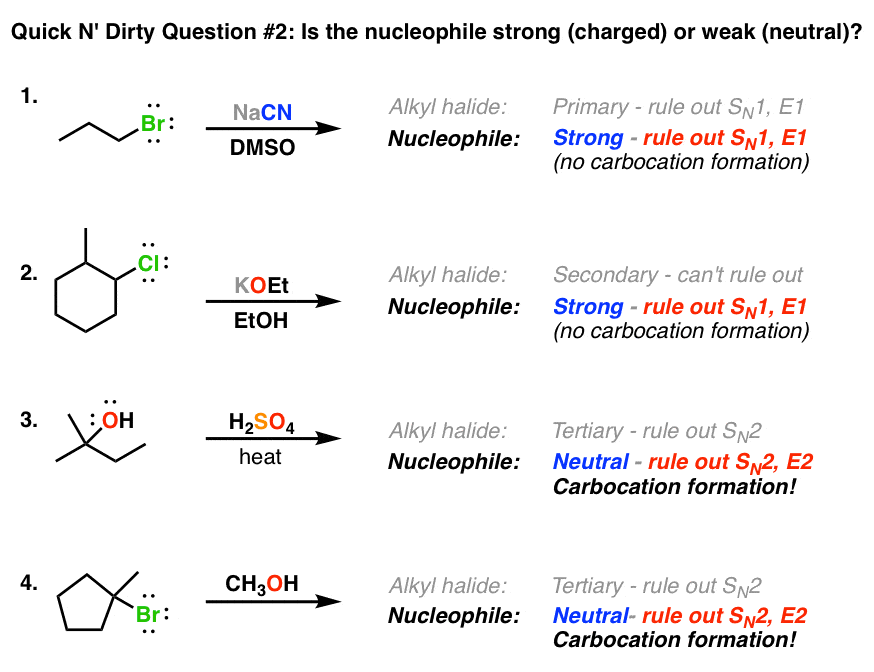
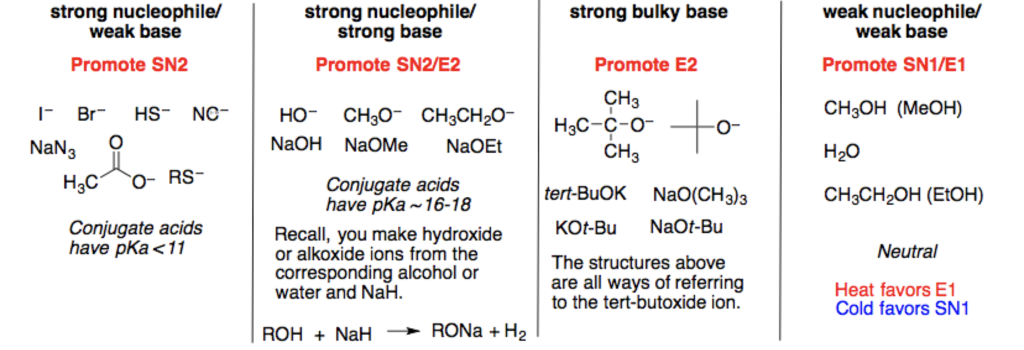
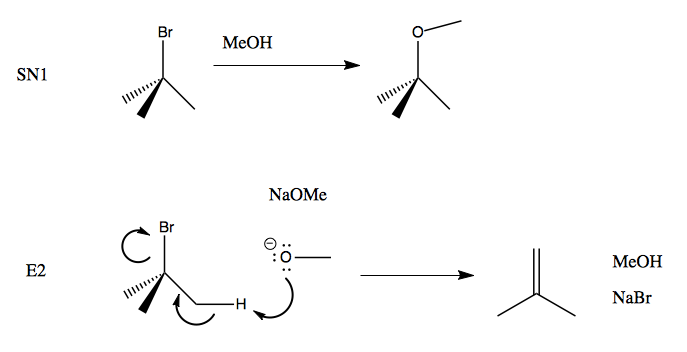
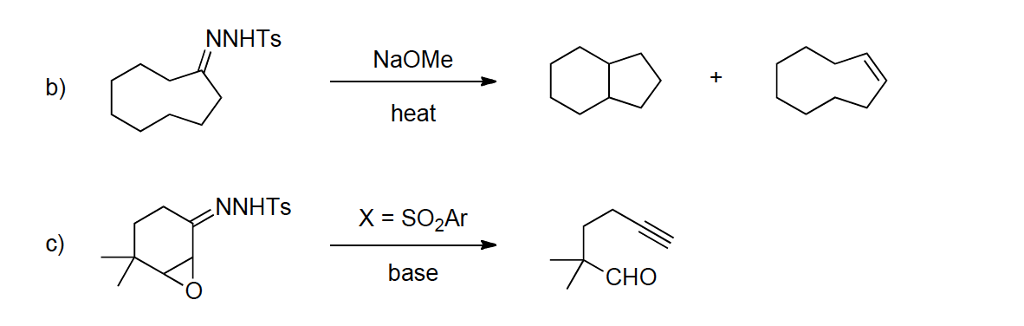
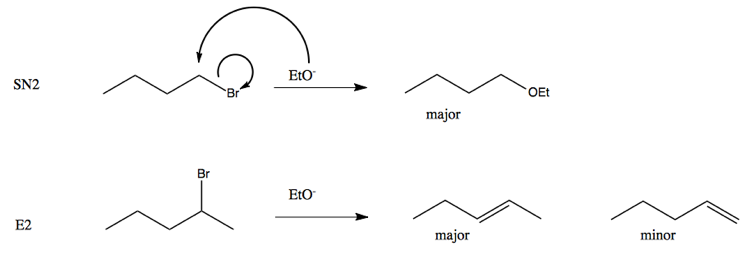
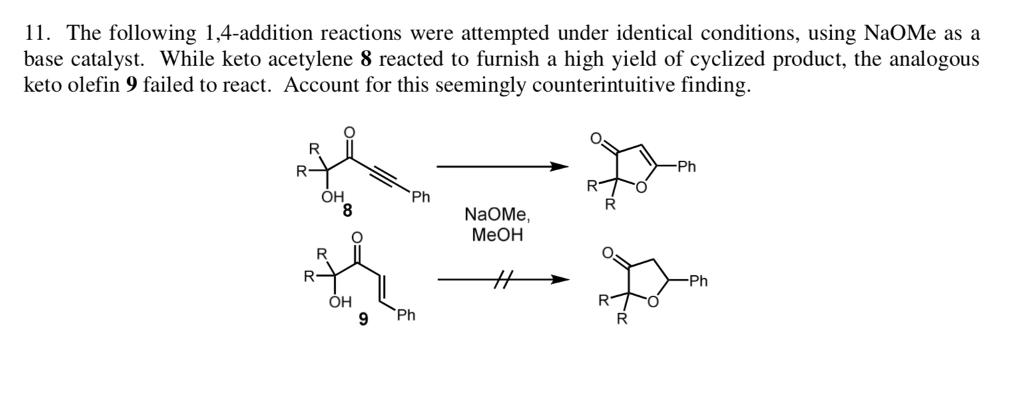
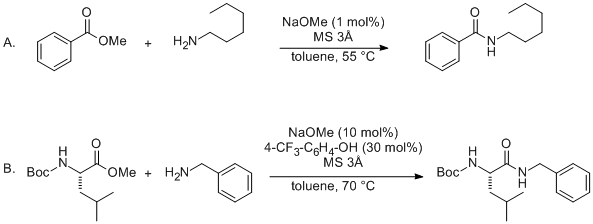
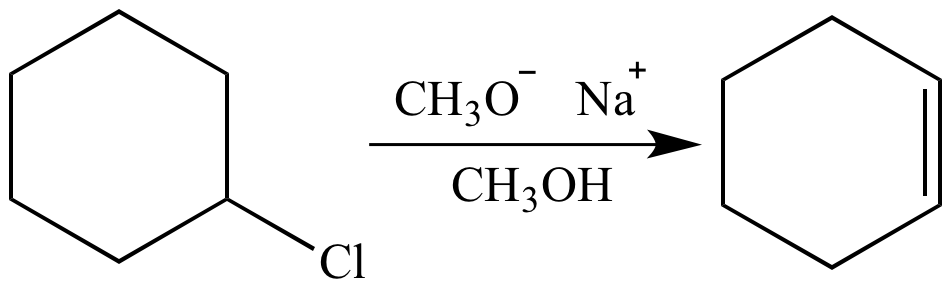Which of the following would be the best base for performing the following elimination? A. NaOH B. NaOMe C. KOtBu D. H2O | Homework.Study.com
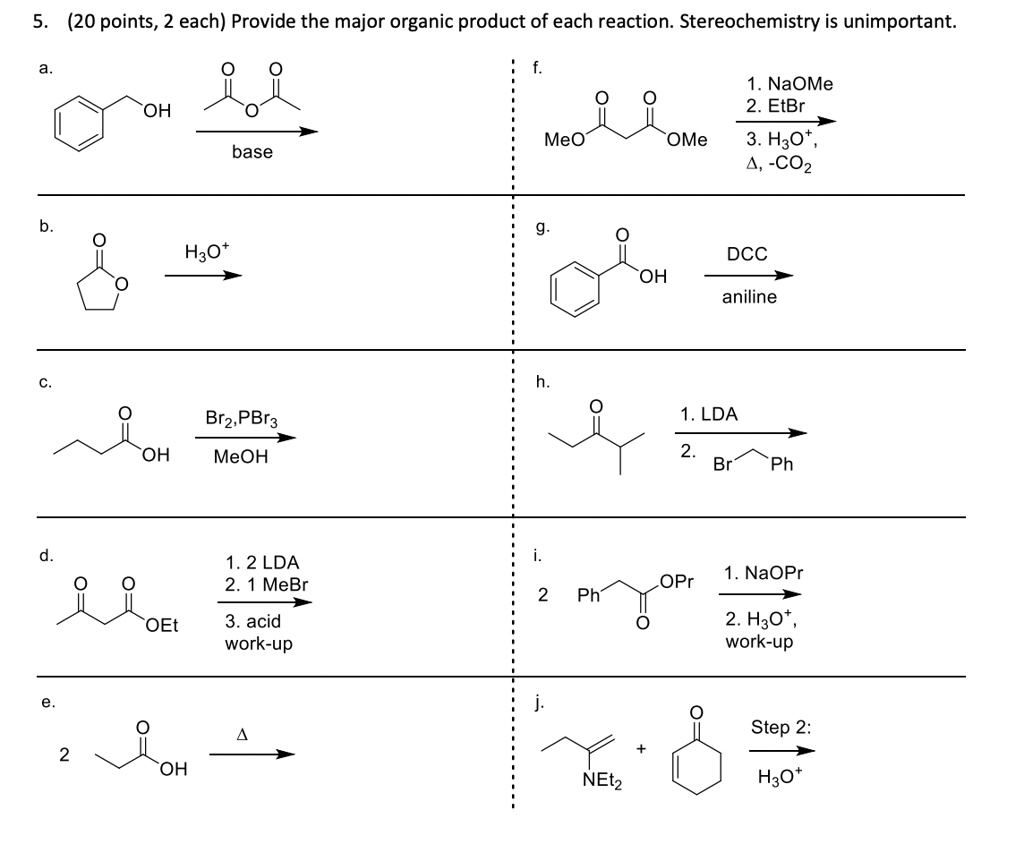
SOLVED: (20 points, 2 each) Provide the major organic product of each reaction. Stereochemistry is unimportant NaOMe 2.EtBr OH Meo OMe 3. H3O COz base Hzot DCC OH aniline 1. LDA OH

Supply structures for H through K. Given : " An Aldohexose "overset (MH2OH"/ base ")(to)H overset (Ae2O"/"NaOAC)(to)I overset (-HOAC)(to)J overset (NaOMe "/"MeOH)(to)K.

organic chemistry - Can the nitrogen of an amide displace a primary chloride in a SN2 reaction in the presence of a strong base? - Chemistry Stack Exchange

Scheme 2. Nucleophilic addition of NaOMe onto 1: acetal formation under... | Download Scientific Diagram

SOLVED: What major product results from the following E2 reaction? NaOMe MeOH Br With the small base of NaOMe, will the base remove a proton from the beta-CH2 or beta-CH when deriving